Abstract
Introduction
B-cell acute lymphoid leukemia (B-ALL) with t(17;19)(q22;p13)/TCF3-HLF is very rare and has a dismal prognosis even with the application of allogeneic hematopoietic stem cell transplantation (allo-HSCT). Due to the rarity of this fusion, only a few cases have been described in the literature. In this study, we retrospectively analyzed 24 cases with TCF3-HLF from a large cohort of B-ALL, which constituted the largest cohort of TCF3-HLF-positive ALL reported to date and reported in detail the laboratory characteristics and prognoses of this group of patients.
Methods
From Apr. 2012 to Feb. 2020, a total of 3287 cases were diagnosed with B-ALL in our hospital. All of them underwent afusion gene screening test including TCF3-HLF through multiplex-nested reverse transcription-PCR. Whole transcriptome sequencing (WTS) was performed using RNA extracted from the bone marrow (BM) samples by HiSeq 2500. The gene expression signature for TCF3-HLF-positive B-ALL was investigated by comparing with healthy controls, B-ALL with TCF3-PBX1/TCF3-ZNF384, and B-ALL negative for pathogenic fusion genes (Chen X et al., Blood Cancer J 2021).
Results
A total of 24 cases with TCF3-HLF were identified, accounting for 0.73% of all B-ALL cases. Among them, 22 (91.67%) were children (≤18 years), and 2 (8.33%) were adults. Length of follow-up varied. The endpoint of the follow-up was Jul. 1st, 2020. Overall survival (OS) was defined as the time from diagnosis to death or the time of the last follow-up.
Of the 24 cases with TCF3-HLF, 8 had type I, 12 had type II, 4 had both type II and III chimera isoforms. Karyotype was available in 22 cases, 19 showed abnormal karyotypes and most of them (12/19, 63%) harbored further structural and/or numerical aberrations besides t(17;19)(q22;p13) translocation. Gene mutation screening of 58 genes was performed on 17 cases at the time of diagnosis. Eight (47%) of them showed mutations and 7 of them had mutations involving RAS signaling pathway genes (NRAS, KRAS, FLT3, and PTPN11). Immunophenotypic examination showed 12 (50%) and 19 (79%) patients exhibited aberrant expression of CD13 and CD33, only 4 patients (17%) were negative of both CD13 and CD33.
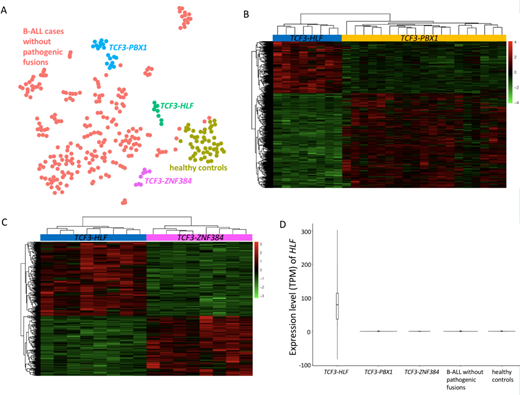
Gene expression clustering revealed apparent separation of TCF3-HLF-positive cases from TCF3-PBX1/TCF3-ZNF384-positive cases and those without pathogenic fusions. The differential expression of 535 genes (up: 207, down: 328) was identified in TCF3-HLF-positive cases compared to TCF3-PBX1-positive cases. The differential expression of 471 genes (up: 281, down: 190) was identified in TCF3-HLF-positive cases compared to TCF3-ZNF384-positive cases. TCF3-HLF-positive patients displayed a significantly up-regulated expression of HLF, which was almost not expressed in other cases (Figure 1).
The median OS of the 24 patients was 18.5 months (range 6-75 months). Thirteen of them underwent allogeneic HSCT (allo-HSCT) and the median OS was 23 months (range 13-75 months). Eight of them were in complete remission (CR) until the last follow-up; 2 of them relapsed after a first allo-HSCT and survived in CR after a second allo-HSCT; 3 of them died (2 died of relapse and 1 died of lung infection under CR). Eleven cases did not receive allo-HSCT, and the median OS was 9 months (range 6-29 months). Seven of them died (6 died after relapse, 1 died without achieving remission); 3 of them relapsed and re-induction was failed; only one case has survived in CR for 19 months till the last follow-up. Twelve cases underwent chimeric antigen receptor T-cells (CAR-T) therapy. Nine of them achieved CR after CAR-T therapy and bridged to allo-HSCT; one case achieved CR after CAR-T therapy but relapsed and lost opportunity for allo-HSCT; the other 2 patients achieved CR after the first application of CAR-T therapy but failed to achieve CR again by CAR-T therapy when relapsed.
Conclusions
We provide systematic insights into the laboratory characteristics and prognoses of B-ALL cases with TCF3-HLF in a large cohort. TCF3-HLF-positive B-ALL has a characteristic gene expression profile that differs markedly from TCF3-PBX1 and TCF3-ZNF384-positive B-ALL and shows a dismal prognosis. TC3F-HLF-positive ALL remains an incurable disease, although CAR-T therapy and allo-HSCT can improve the prognosis to some extent. Advanced therapeutic approaches, including novel drug discovery and development, are urgently required to improve the outcome of this ALL subtype.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal